This article was originally printed in the May/June 2025 issue of the California Veterinarian magazine.
Drug misbranding is a violation identified by the California Veterinary Medical Board (CVMB) during complaint investigations and routine practice inspections. Many veterinarians may unknowingly be noncompliant, as this topic is minimally covered in primary and continuing veterinary education. But in fact, several federal and state laws address drug misbranding and are directly applicable to veterinary practice. This article outlines key regulations, common violations, and tips for compliance.
Drug Misbranding Legal Definition
Although drug misbranding encompasses several laws and takes many forms, California Health and Safety Code section 111330 provides the easiest definition to help veterinary practices decipher whether or not a drug is misbranded: “Any drug or device is misbranded if its labeling is false or misleading in any particular.” “Particular” is used as a noun in this definition and is synonymous with the word “detail.” In general, misbranded drugs lack approval from the federal Food and Drug Administration (FDA).
Drug Misbranding in Veterinary Practices
While several forms of drug misbranding are prevalent in veterinary practice, two stand out above the rest:
- Using and/or selling a drug with a false or misleading label (often lacking FDA approval).
- Improperly repacking or relabeling an FDA-approved drug for in-house use or client dispensation.
What About Compounded Drugs?
If no suitable FDA-approved drug is available, a veterinarian may have that drug compounded by a compounding pharmacy for extra-label use or may compound it themselves within the parameters set forth in the law. While compounded drugs are technically misbranded by legal definition, the FDA exercises enforcement discretion in their regard. The CVMB also recognizes that compounded drugs are a special component of veterinary drug inventory that must be enforced by different labeling rules than those set forth for drug misbranding.
Drugs compounded in a registered veterinary premises must follow Title 16, California Code of Regulations (CCR), sections 2090–2095. Per section 2094(b), labels must include:
- Name, strength, and quantity of each ingredient
- Expiration date
- Lot number or control number assigned by the preparer
- Name or initials of the preparer
- Date of drug preparation
 Image 1: Misbranded drugs compounded within a veterinary practice
Image 1: Misbranded drugs compounded within a veterinary practice
Veterinarian’s Legal Liability
Purchasing from a distributor or manufacturer does not guarantee that a drug is not misbranded. Veterinarians are legally responsible for ensuring drugs used or dispensed are not misbranded. While a myriad of misbranding laws exist, key laws include:
- California Health and Safety Code section 111440.
This law states that “it is unlawful for any person to manufacture, sell, deliver, hold, or offer for sale any drug or device that is misbranded.” Therefore, if a veterinarian has in their possession for use in practice or for sale any misbranded drug, they are in violation of the law. - California Business and Professions Code section 4169.
This law declares that a person cannot purchase, trade, sell, or transfer prescription drugs that the person knew or reasonably should have known were misbranded. This means that a veterinarian must ensure that the drugs they purchase are not misbranded. - California Business and Professions Code section 4883(g)(3).
This law states that the CVMB may deny, revoke, or suspend a license or registration or assess a fine for unprofessional conduct, which includes but is not limited to a violation of any federal or state statute, rule, or regulation governing drugs (including controlled substances). Therefore, the CVMB has authority to investigate and inspect drugs in a veterinary practice, determine if a misbranding violation exists, and levy discipline on a veterinarian for applicable violations.
Examples of Misbranding
- A label placed on a drug used within the veterinary practice must contain the same information as the original label it is covering.
- A dispensation label (for drugs that are being sent home with clients from the veterinary practice stock) must contain information specified in 16 CCR, section 2032.2(b). The dispensation label may cover the original label but must contain all of the information specified in the aforementioned code in order to remain in compliance.
- Drug mimics (copies) an FDA-approved drug and/or is manufactured in a non-FDA-registered facility.
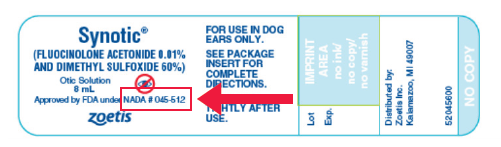
The name of the FDA-registered drug manufacturer must be written on the label. FDA-approved drugs will bear “A New Animal Drug Application” (ANADA or NADA) number on the label. To determine if the manufacturer is properly registered with FDA to produce the drug, search the FDA Greenbook.
Dispensation Labels
Dispensing a drug means that it is being taken out of the veterinary practice stock and labeled to give to the client. Whether it is in its original packaging or being parsed into a secondary container, a dispensation label must be affixed to comply with 16 CCR section 2032.2(b). The label contents must include:
- Name, address, and telephone number of the facility
- Client’s name
- The species and name, number, or other identifying information for the animal
- Date dispensed
- Directions for use, including, if applicable, withdrawal time
- The manufacturer’s trade name of the drug or the generic names, strength (if more than one dosage form exists), quantity of drug, and the expiration date when established by the manufacturer
- Name of prescribing veterinarian
Per Title 21 of the Code of Federal Regulations, section 290.5, any schedule II–IV controlled substance dispensation label must state:
“Caution: Federal law prohibits the transfer of this drug to any person other than the patient for whom it was prescribed.”
When dispensing individually drawn syringes that cannot accommodate their own label, place the syringe(s) into a labelled drug vial with a child-proof cap.
For tablets that are halved or quartered, indicate that they have been halved or quartered on the label to assist the user with understanding the prescribed dose.
 Image 3: Drug mimics (copies) an FDA-approved drug and/or is manufactured in a non-FDA-registered facility
Image 3: Drug mimics (copies) an FDA-approved drug and/or is manufactured in a non-FDA-registered facility
Tips to Avoid Misbranding Violations
To remain compliant with federal and state misbranding laws, veterinarians are obligated to verify that the drugs they are administering and dispensing to patients are not misbranded.
- Look for the “A New Animal Drug Application” (ANADA or NADA) number on the drug container. Unless the drug is counterfeit or fraudulent, the presence of this number on the container is a good indicator that it is FDA-approved.
- Don’t confuse a National Drug Code (NDC) number with the ANADA number. The NDC number only indicates that the FDA is aware of a drug’s existence. It does not mean that the drug has undergone safety and efficacy testing for the label claim or label components to receive FDA approval.
- Routinely look up drugs in the FDA Greenbook. The FDA Greenbook is a current list of all Approved Animal Drug Products and is updated monthly. The FDA offers the public an online database searchable by the drug trade name or the generic name. Access the FDA Greenbook.
- When in doubt, contact the drug manufacturer. An FDA-approved drug will have the name of the manufacturer on the label (the absence of such information is confirmation of misbranding). The drug manufacturer should be able to confirm that the drug is FDA-approved.
- Properly label all drugs used in house and dispensed to clients. Follow the labeling requirements for secondary drug containers used in the veterinary practice and for drugs dispensed from practice stock to clients. Instructions for both are provided above.
Drug misbranding can compromise patient safety and lead to serious legal consequences. Veterinarians have a legal responsibility to understand drug misbranding and ensure that all drugs in their practice are lawful to possess and are properly labeled.







 Image 1: Misbranded drugs compounded within a veterinary practice
Image 1: Misbranded drugs compounded within a veterinary practice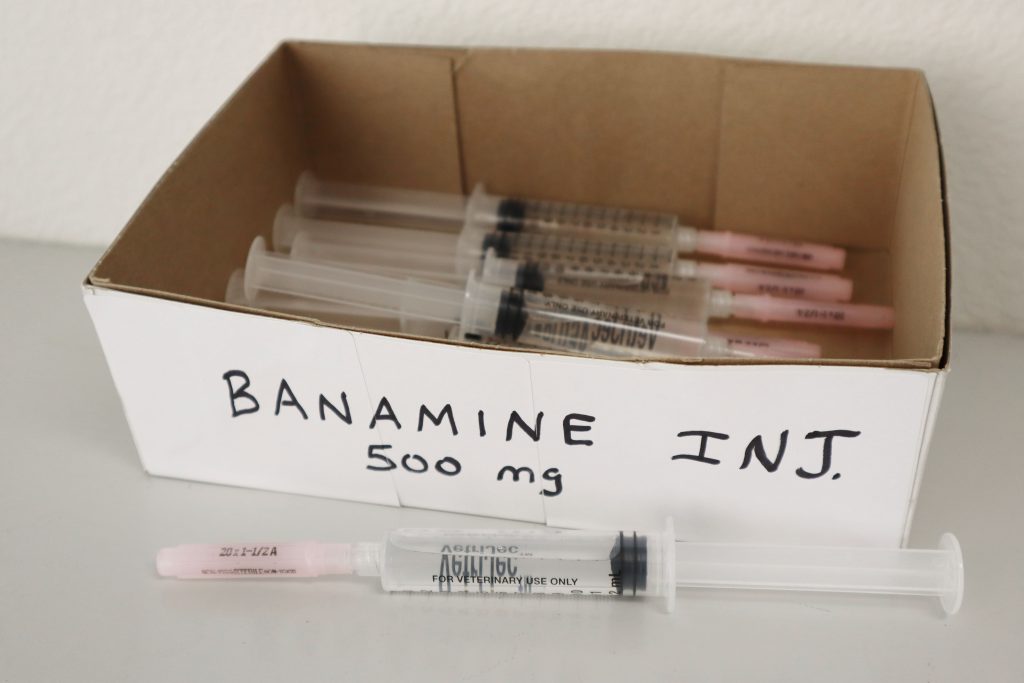 Image 2: Loose capsules/tablets or pre-loaded syringes without labels
Image 2: Loose capsules/tablets or pre-loaded syringes without labels Image 3: Drug mimics (copies) an FDA-approved drug and/or is manufactured in a non-FDA-registered facility
Image 3: Drug mimics (copies) an FDA-approved drug and/or is manufactured in a non-FDA-registered facility
